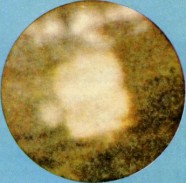
20 ng of berkelium oxide. (1)
99 - Berkelium

20 ng of berkelium oxide. (1)

Berkelium compound visible as the small
gold spot (arrow). (2)

First berkelium metal sample (about 1.7 ug). (3)
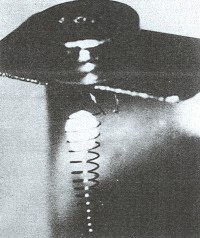
BkF4 in a tungsten spiral.
This is reduced with lithium vapor to yield berkelium metal.
(4)
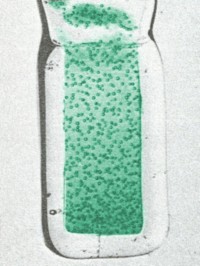
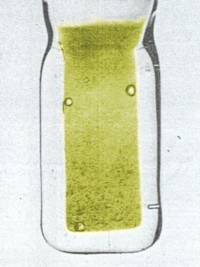
Simulated colors of Bk (III) hydroxide [left] and Bk (IV) hydroxide [right].
The original pictures were printed in black and white, with color descriptions as
'mint green' and
'bright yellow'. I colorized the black and white pictures
to try and approximate this description.
The bubbles in the Bk (III)
picture are due to the reducing agent needed to keep this oxidation state.
Otherwise, it decomposes.
(5)
1 - "Matter", Ralph E. Lapp and the editors of LIFE, LIFE
Science Library, 1965.
2 - "Matter", Ralph E. Lapp and the editors of LIFE, LIFE
Science Library. A newer edition than 1965, I
don't know which year.
3 - Copied from "Die Metalle", p. 74. Photo credited to Oak Ridge National
Laboratory. Thanks to David Keim for the reference.
4 - R. G. Haire, "Preparation of transplutonium metals and
compounds", p 309-342, printed in "Actinides in Perspective", N.M. Edelstein
ed., Pergamon
Press, 1981.
5 - D. Cohen, "Oxidation-reduction reactions of the
transuranium elements", J. inorg. nucl. Chem., Supplement 1976.
Printed in "Proceedings of the Moscow symposium on the Chemistry of
Transuranium Elements", V. Spitsyn, J. Katz ed., Pergamon Press, 1972.