
2.5 grams of radium bromide in a weighing tube. Radium compounds glow in the dark due to the strong radioactivity. (1)
88 - Radium

2.5 grams of radium bromide in a weighing tube. Radium compounds glow in the
dark due to the strong radioactivity. (1)
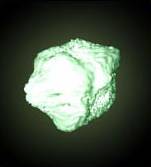
Radium metal, freshly prepared. (It turns black upon exposure to air.)
(2)


Radium bromide mixed with zinc sulfide - a mixture used in luminous watch dials.
The radium gives off dangerous radiation which causes the zinc sulfide to glow.
(3)

Radium
containing watch hands. Similar hands are actually obtainable by
individuals. (4)
1 -
http://www.chemie-master.de/. Deutsches Museum München,
http://www.deutsches-museum.de/
2 - American Institute of Physics,
http://www.aip.org/history/curie/brief/03_radium/radium_8.html
3 - "Matter", Ralph E. Lapp and the editors of LIFE, LIFE
Science Library, 1965.
4 - eBay seller just4me53,
http://myworld.ebay.com/just4me53/